Abstract
Background: Systematic guidance for considering health equity in guidelines is lacking. This scoping review aims to synthesize current best practices for integrating health equity into guideline development and the benefits or drawbacks of these practices.
Methods: We searched Ovid MEDLINE ALL and Embase Classic+Embase on the Ovid platform, CINAHL on EBSCO, and Web of Science (Core Collection) from 2010 to 2022. We searched grey literature from 2015 to 2022, using the Canadian Agency for Drugs and Technologies in Health Grey Matters checklist and searches of potentially relevant websites. Articles were screened independently by 1 reviewer. Proposed best practices, advantages and disadvantages, and tools were extracted independently by 1 reviewer and qualitatively synthesized based on the relevant steps of a comprehensive checklist covering the stages of guideline development.
Results: We included 26 articles that proposed best practices for incorporating health equity within the guideline development process. These practices were organized under different stages of the development process, including guideline planning, evidence review, guideline development and dissemination. Included studies provided best practices from guideline producers, articles discussing health equity in current guidelines, articles addressing strategies to increase equity in the guideline implementation process, and literature reviews of promising health equity practices.
Interpretation: Our scoping review identified best practices to incorporate health equity considerations at each phase of guideline development. Identified practices may be used to inform equity-promoting strategies with the guideline development process; however, guideline producers should carefully consider the advantages and disadvantages of best practices when integrating health equity.
The attainment of the highest possible standard of health for all is a fundamental human right.1 Over the past 2 decades, many countries and global organizations have undertaken measures to reduce health inequities, 2–4 which are defined as avoidable differences in health that are considered unfair and unjust but modifiable.5,6 Factors that contribute to unfair and avoidable differences in health are diverse, complex and interdependent.7 Populations that are marginalized owing to social, economic or environmental factors may face a higher burden of disease or poorer health outcomes due to structural inequities that result in an unequal allocation of power and resources.7,8 These issues may be further compounded because of a differential ability (or opportunity) to access or use the full spectrum of health care.9 For these reasons, health equity has been increasingly recognized as a vital consideration in clinical practice, public health and policy-making.2,10–13 These issues have been thrown into prominence by the COVID-19 pandemic.14
Evidence-based clinical practice guidelines have the potential to reduce health inequities and improve care among disadvantaged populations.15–17 Guidelines can also unintentionally create or exacerbate existing health inequities between populations.15,17–20 For example, guidelines may recommend a treatment that is inaccessible to the socioeconomically disadvantaged. This could increase the health of those who are more socioeconomically advantaged more readily than those who are disadvantaged, thus widening health disparities.21 Indeed, guidelines that solely consider evidence of effectiveness of clinical options as a foundation for the recommendations without consideration of the evidence related to their implementation, acceptability, feasibility and capacity to mitigate disparities do not meet international standards of quality.22 Incorporating health equity into clinical practice guidelines remains a challenge because there is no widely accepted guidance or standard for reporting quality and the few available tools or checklists for evaluating guideline quality do not include health equity.16,23 The impact of the pandemic on health care systems is likely to create an urgent demand for guidelines to address the accumulated need for health care decisions, and a need for these decisions to consider health equity.24–26
In 2014, a content analysis was performed to outline methodological themes and processes on how to address health equity in guideline development.27 Since 2014, several guideline developers (e.g., the National Institute for Health and Care Excellence in the United Kingdom, the National Health and Medical Research Council in Australia28 and the World Health Organization29) have updated their guidance for considering health equity in guidelines. Notably, in 2017 the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group released a series of papers providing guidance on how to explicitly address health equity in the GRADE guideline development process.17 Whereas evidence has accumulated over recent years, no reviews, to our knowledge, have synthesized contemporary strategies to incorporate health equity into guideline development or compared differences across proposed strategies. Therefore, the objective of this scoping review is to identify current best practices to integrate health equity into guideline development and the benefits or drawbacks of these practices.
Methods
The protocol for this scoping review was made available on the Open Science Framework before the start of the project (https://osf.io/skvnx/). The complete report is also available on the Open Science Framework. Levac and colleagues’ update of the Arksey and O’Malley methodological framework for scoping reviews guided this review.30,31 We also followed the methodology manual published by the Joanna Briggs Institute for scoping reviews,32,33 where applicable. Given budgetary and timeline restraints, we selected a rapid review methodology whereby components of the scoping review are simplified or omitted (e.g., 1 reviewer) to produce results in a timely manner.34,35
Literature search
An experienced medical information specialist (B.S.) developed and tested the search strategies through an iterative process in consultation with the review team. Another senior information specialist peer reviewed the strategies before execution using the Peer Review of Electronic Search Strategies checklist (Appendix 1, available at www.cmajopen.ca/content/11/2/E357/suppl/DC1).36 Using the multifile option in Ovid, we searched Ovid MEDLINE ALL and Embase Classic+Embase. We also searched CINAHL (EBSCO) and the Web of Science (Core Collection). All searches were conducted on Nov. 23, 2020, and updated on July 30, 2022. Strategies used a combination of controlled vocabulary (e.g., “Guidelines as Topic”) and guideline-related keywords in proximity to terms representing either processes (e.g., develop, framework, process) or disadvantaged populations (e.g., disparity, inequity, underserved). Vocabulary and syntax were adjusted across databases. Where possible, animal-only and opinion pieces were removed from our searches using filters. There were no language restrictions applied to our search strategy, but search results were limited to publication dates from 2010 onwards for feasibility purposes (e.g., time and budget constraints). An initial scan of the published literature showed that sources of interest were published after 2010. Results were downloaded and deduplicated using EndNote version 9.3.3 (Clarivate Analytics). The full strategies can be found in Appendix 2, available at www.cmajopen.ca/content/11/2/E357/suppl/DC1.
We conducted a targeted search of the grey literature to identify relevant nonindexed and unpublished literature using the Canadian Agency for Drugs and Technologies in Health Grey Matters checklist37 and thorough searches of potentially relevant websites (Appendix 3, available at www.cmajopen.ca/content/11/2/E357/suppl/DC1). Grey literature searches were limited to English language documents published from 2015 to 2022. A more recent cut-off (2015) was selected for grey literature to limit the amount of grey literature to be screened and conduct a more comprehensive search of materials issued in recent years.
Study eligibility criteria
Table 1 outlines the study inclusion and exclusion criteria. Relevant studies were included if they described procedures or processes that address health equity in the guideline development process. Articles that described equity promotion practices in primary research studies (e.g., promoting health equity when conducting randomized clinical trials) or systematic reviews (only) were excluded. Only studies published in English or French were included. Eligible study designs included primary research designs, reviews or guidelines. Commentaries, editorials, responses, opinion pieces, protocol registrations and animal-only studies were excluded.
Eligibility criteria
Study selection
The article selection process consisted of 2 phases of screening: title and abstract review and full-text review. After the removal of duplicates, the titles and abstracts of all references identified in our search were uploaded into Covidence software (Covidence) for screening.38 A pilot screening exercise occurred before each phase of screening to ensure interrater reliability and determine the adequacy of the screening criteria. For both phases, articles were screened by a single reviewer (N.S. or A. Bennett) using the eligibility criteria described above. An additional second reviewer (N.S., A. Bennett or A. Beck) verified included references and performed a secondary review of any references that the first reviewer was unsure met the inclusion criteria.
Charting the data
All included full-text articles were reviewed and charted by 1 reviewer using a pilot-tested data extraction form. Data extraction was completed using NVivo Software released in March 2020 (QSR International).39 The data extraction template is available in Appendix 4 (www.cmajopen.ca/content/11/2/E357/suppl/DC1). We captured data items related to study characteristics, including author and organization, study design, the article’s aim, and a description of the population and setting. When articles provided equity recommendations specifically for guidelines, we extracted best practices and mapped them to the stages of the guideline development process, as outlined by a comprehensive guideline development checklist.40 The benefits and drawbacks of these approaches, as described by the authors, were also extracted if available. We did not formally appraise the methodological quality of included articles, as our primary goal was to map any available evidence, either from the peer-reviewed or grey literature, rather than identify the highest-quality evidence to answer a specific key question related to policy or practice.32
Data analysis
Identified best practices are mapped against a comprehensive guideline development checklist that outlines 18 topics for guideline development.40 Tables are included to summarize included study characteristics, practices for incorporating equity in guideline development, advantages or disadvantages of these practices, and relevant frameworks and tools.
Ethics approval
We did not require ethics approval for this study.
Results
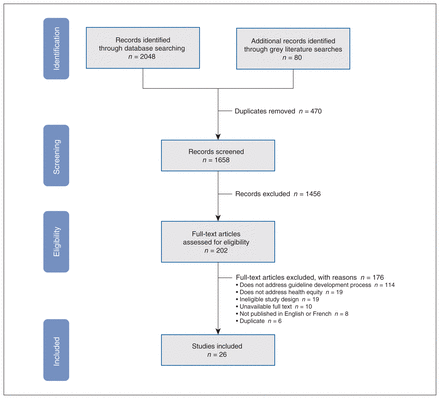
A total of 26 articles proposed best practices for incorporating health equity within guideline development. Study characteristics are presented in Table 2. Full texts were excluded because they did not address the guideline development process (n = 114) or describe practices for the promotion of health equity (n = 19); other reasons were ineligible study designs (n = 19), ineligible language of publication (n = 8), unavailable full texts (n = 10) or duplicate articles (n = 6). The screening process is summarized in our PRISMA flowchart (Figure 1), and the list of excluded studies at full-text screening can be found in Appendix 5 (available at www.cmajopen.ca/content/11/2/E357/suppl/DC1).
Characteristics and summary of included articles, grouped by organization
PRISMA flow diagram and list of excluded full-text studies with reasons.
Five articles focused on a specific population or subgroup including indigenous populations,50 individuals with intellectual disabilities,20 minority ethnic groups,48 individuals with lived experience of homelessness,58 and gender groups.57 Key sources included the GRADE equity guideline series published in 2017, which provided guidance and examples on considering equity at key stages of the guideline development process.17,41–43 Shi and colleagues conducted a review (published in 2014) synthesizing methods for incorporating equity in clinical practice guidelines.27 Other articles included sources that provided best practices from guideline producers, 28,29,46,48,50,51 articles discussing health equity in current guidelines,2,20,52,53,56 articles addressing strategies to increase equity in the guideline implementation process,15,44,58 and literature reviews of health equity practices.47,57
Table 3 provides a detailed summary of best practices, their advantages and disadvantages, and any relevant tools for health equity promotion. We structured the results using the relevant topics of a comprehensive checklist covering the stages of guideline development40 and further organized topics under 4 phases: guideline planning, evidence review, guideline development and dissemination. Of the 18 topics outlined in the comprehensive checklist for guideline development, we identified 12 topics where proposed best practices for incorporating health equity can be considered. “Assessing equity within guidelines” was an additional topic under guideline development we identified through our synthesis.
Summary of proposed best practices within each of the 4 stages of guideline development
In some cases, similar strategies and tools for health equity promotion were identified by several different included sources. For example, 4 unique articles noted that PROGRESS-Plus60 may help guideline developers systematically consider and prioritize populations for whom the health care topic is relevant. 27,28,41,56 Six sources suggested including representatives and stakeholders from disadvantaged groups who are involved throughout the entire guideline development process.20,28,41,44,50,51
We detected no incompatibility among the identified unique strategies for each guideline topic. However, for some guideline topics where multiple strategies were suggested, only 1 strategy was recommended to be selected for implementation. For example, the New Zealand Guideline Group’s framework50 presented 2 alternative approaches to promoting indigenous (Māori) representation in guideline group membership. One approach was to include representatives of disadvantaged population groups in the guideline group and the other was to create an independent subgroup of representatives within the larger guideline group. Either of these approaches were suggested to promote indigenous representation, and each had associated advantages and disadvantages (Table 3).
Interpretation
Our scoping review found substantive recommendations on best practices to incorporate health equity during the 4 phases of guideline development (Table 3). Of the 18 topics outlined by the comprehensive checklist for guideline development, we identified 12 topics where incorporating health equity can be considered. We identified an additional topic under guideline development for “assessing equity within guidelines.” We included 26 articles from peer-reviewed and grey literature sources, including reports from federal and provincial agencies, community health centres and international guideline producers.
Since the systematic review by Shi and colleagues published in 2014,27 several articles have been published, including the GRADE equity series, which provided comprehensive guidance and real-world examples regarding equity promotion. Our review captured new articles and additional sources related to health equity promotion, including health equity toolkits, interventions to increase equity in primary care delivery and organizational health equity plans. One article, published in 2020, highlighted the importance of increasing awareness of gender-specific medicine in tailored guidelines to properly address characteristics and needs of certain populations within each gender.57 Gender differences of diseases is a neglected dimension of medicine and not included in most guidelines.57,70 Additionally, improving the care for marginalized populations, including individuals experiencing homelessness, can be achieved through identifying determinants of guideline implementation.58 One study identified a number of specific knowledge translation strategies in the context of individuals experiencing homelessness, through a qualitative survey, that may affect the implementation of guidelines, including homeless-specific training for health professionals, feasibility of permanent supportive housing and discrimination faced by those with lived experience of homelessness.58 These practical examples illustrate considerations guideline developers can acknowledge when looking to promote health equity in guideline development.
Although equity-related guidance was captured for most stages of guideline development,40 some gaps in the knowledge base remain. No equity-related guidance was captured to identify or report on conflicts of interest, an important consideration for clinical guideline producers because of potential vulnerability from industry influence.71 We identified few strategies and tools relating to equity promotion in guideline reporting, peer review, monitoring guideline uptake and updating. Additionally, there was no discussion on the advantages or disadvantages of best practices for the final stages, dissemination and uptake of recommendations. Future research may need to explore whether special considerations related to equity are required for these steps in the guideline process.
Some of the identified strategies have become more commonplace in evidence reviews and the guideline process, in general. For example, some of the guidance we found related to “searching for relevant evidence” were applied in our own scoping review search strategy. This included not adding language filters to our search strategy, considering evidence from qualitative and observational studies, and screening evidence outside of traditional health sources in our grey literature search strategy. However, despite the evidence base on health equity in guideline development existing since 2011,72 the uptake of health-equity promoting practices is slow. A review of WHO guidelines published between 2014 and 2019 found that only 54% of guidelines used the Evidence to Decision framework to consider health equity and that only 28% of recommendations from these guidelines related to health equity were supported by research evidence.73
Guideline producers should consider the use of guideline checklists and tools to implement health-equity promoting practices throughout guideline development. The strategies synthesized in this scoping review may help in supporting guideline organizations to develop their own health equity framework or plan. However, the selection of equity-promoting strategies must be tailored to the goals of a particular guideline organization. For example, some equity practices targeted toward WHO clinical guidelines may be less relevant for clinical practice guidelines for primary care practitioners. Additionally, we limited our discussion of advantages and disadvantages to those that had been identified in the original articles. There may be additional benefits or limitations to practices when considering implementation. For example, one strategy when identifying a guideline’s target audience was to involve representatives of disadvantaged groups.41 Whereas no drawbacks were discussed in the article, implementing such a practice would likely have cost and resource implications for the guideline organization, from additional time needed to contact and secure appropriate representatives and to compensate representatives for their time. Finally, developers should remain conscious of important systemic health and social inequities in our health care system when implementing practices. Clinical and epidemiologic research has highlighted the dangers of “othering” certain patient groups. The provision of separate medical care or recommendations for population subgroups, such as in race-based medicine, may further exacerbate health disparities rather than mitigate them.74,75 Any equity framework or plan should be developed in partnership with experts in the field of health equity, as well as health system stakeholders and community organizations.
Limitations
Given the urgency of the need for a map of equity considerations in guideline development and resource restraints, a rapid scoping review methodology was selected as the best available approach to answer our research question; however, limitations to our methodology should be acknowledged. Although our search strategy was comprehensive, we may have failed to capture articles on equity-promoting strategies using a single reviewer, especially if these studies did not explicitly define these strategies (e.g., tools to facilitate patient engagement). To mitigate this concern and validate our search strategy, we consulted an external content expert in health equity to review our excluded studies list. Although this is a limitation and may have resulted in relevant practices being missed, it is unlikely to bias our results, as our goal was to provide a summary of best practices. Finally, a narrative synthesis was used to analyze and summarize our results. Efforts were made to be systematic in our use of qualitative data synthesis methods; however, we did not follow a formal thematic content analysis process, which may reduce our review’s reproducibility.
Conclusion
Overall, our scoping review found considerable evidence on proposed best practices to promote health equity. Identified practices may be used to inform equity-promoting strategies within the guideline development process and within the guideline organization itself. Whereas health equity is a complex issue and guideline organizations must carefully balance the pros and cons of best practices, our review provides an overview of available strategies and resources to aid guideline producers in creating a plan to integrate health equity in a timely way.
Footnotes
↵* Dr. Ainsley Moore died June 25, 2021.
Competing interests: Navindra Persaud reports grants from the Canadian Institutes of Health Research, Canada Research Chairs Program and the Ontario SPOR Support Unit. He is a member of the Canadian Task Force on Preventive Health Care (travel expenses paid for by the Public Health Agency of Canada), advisor to the pan-Canadian Advisory Panel on a Framework for a Prescription Drug List (no payment or compensation) and an associate editor for CMAJ. No other competing interests were declared.
Contributors: Nicole Shaver and Alexandria Bennett contributed to conceptualization, project administration, methodology, writing the original draft and revisions. Andrew Beck contributed to the methodology, writing the original draft and revisions. Becky Skidmore reviewed and edited the manuscript and performed the search strategy. Melissa Brouwers, Julian Little and David Moher acquired funding, contributed to the methodology, and reviewed and edited the manuscript. Gregory Traversy, Ainsley Moore and Navindra Persaud conceived, reviewed and edited the manuscript. Nicole Shaver and Alexandria Bennett contributed equally to this work. All authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: Funding for this evidence review is provided by the Public Health Agency of Canada. The views expressed herein do not necessarily represent the views of the Public Health Agency of Canada.
Data sharing: All data presented are available in the published record, and the protocol and full report are available on the Open Science Framework (https://osf.io/skvnx/).
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/11/2/E357/suppl/DC1.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/
References
- © 2023 CMA Impact Inc. or its licensors